The Tydeman® Tube – An Innovative Medical Device to Facilitate Delivery of the Impacted Fetal Head at Caesarean Section

Wednesday 24 July, 2024WASHINGTON, UK (24th July, 2024) - Rocket Medical Plc. and Guy’s and St Thomas’ NHS Foundation Trust are pleased to confirm the launch of the Tydeman® Tube, an innovative medical device that is used to aid in the delivery of the impacted fetal head at caesarean section. The device was invented by NHS Fife consultant obstetrician Dr Graham Tydeman, with the aim of improving outcomes for patients and babies.
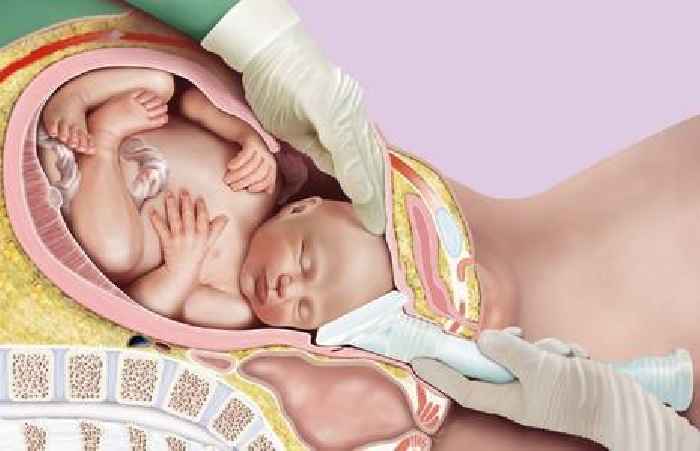
Impacted fetal head at caesarean section is a serious and challenging situation which has been encountered by the large majority of obstetricians[1]. In these cases, the baby’s head is lodged tightly in the pelvis, and in order to deliver the baby, the head must be elevated.
Elevation is commonly achieved by asking an assistant to push the head up from below. Considerable force is often needed and this can have serious adverse outcomes for the patient and/or the baby. Injuries may include maternal trauma and haemorrhage, fetal eye injury, skull fracture or even death. NHS Resolution have identified impacted fetal head and/or difficult delivery of the head as an emerging clinical issue, being encountered in 9% of a sample of cases where NHS Resolution panel solicitors were instructed to investigate liability[2].
The Tydeman Tube features a soft cup, which is applied to the baby’s head prior to a push up, in place of the fingertips. A hollow handle assists the user to achieve sufficient elevation. The design aids delivery by spreading the pushing force across a larger surface area versus manual push up with the fingers[3], as well as relieving any suction effect that may be present. Using this tool, the surgeon can direct the assistant as to the amount and direction of force to be applied. While many cases of impacted fetal head occur at full dilation, it is also common prior to this[4]. The Tydeman Tube can be used from 7cm dilation onwards.
Initial feedback suggests that the device is easy to use and effective[3]. Professor of Maternal and Fetal Health, Andrew Shennan of King’s College London and Guy’s and St Thomas’ NHS Foundation Trust, trialled the device and said, “We have used the Tydeman Tube at St Thomas’ Hospital and our senior clinicians have found it extremely useful in cases of suspected or encountered impacted fetal head at caesarean section.”
Rocket Medical Managing Director, Daniel J. Agustus, PhD., said, “We are delighted to have partnered with Guy’s and St Thomas’ NHS Foundation Trust to bring the Tydeman Tube to market. At Rocket Medical we strive to improve patient’s lives, and we believe that this device will make a difference to users, patients and babies at what can be difficult and stressful deliveries.”
Dr Debra Guest, Head of IP Commercialisation at Guy’s and St Thomas’ Centre for Innovation, Transformation and Improvement (CITI) said, “Partnering with Rocket Medical has been incredibly positive and we are delighted to launch the Tydeman Tube, which we believe will make emergency C-sections safer. At CITI we are proud to support clinicians to develop solutions to healthcare’s most pressing problems and to work with industry partners like Rocket Medical to make those ideas a reality.”
Jo Norris, Innovation Specialist, NHS Supply Chain said, "Being able to bring new products to clinicians through tangible pathways is one of the key drivers of our innovation offering. Bringing this product to market will further help surgeons during caesarean sections to safely deliver babies in cases of impacted fetal head.”
Tydeman Tube will initially be available in the UK via Rocket Medical and NHS Supply Chain, with additional regions to follow.
For further information please visit www.rocketmedical.com
or contact customerservices@rocketmedical.com
About
The Tydeman Tube was invented by consultant obstetrician Graham Tydeman, NHS Fife and developed in conjunction with Prof. Andrew Shennan, King’s College London and Guy’s and St Thomas’ NHS Foundation Trust, and Prof. Annette Briley, Flinders University and Northern Adelaide Local Health Network (formerly of Guy’s and St Thomas’ NHS Foundation Trust), supported by Guy’s and St Thomas’ Centre for Innovation, Transformation and Improvement (CITI) and the Guy’s and St Thomas’ Charity.
The Tydeman Tube is manufactured and sold by Rocket Medical, Plc. under license from Guy’s and St Thomas’ NHS Foundation Trust.
Rocket Medical Plc is a global designer, manufacturer and distributor of medical devices, specialising in Women’s Health and Drainage.
Guy's and St Thomas' provides 2.6 million patient contacts in acute and specialist hospital services and community services every year. The Trust includes Guy’s Hospital, St Thomas’ Hospital, Evelina London Children’s Hospital, Royal Brompton Hospital, Harefield Hospital, and adult and children’s community services in Lambeth and Southwark.
As one of the biggest NHS trusts in the UK, with an annual turnover of £2.8 billion, Guy's and St Thomas' employs around 25,300 staff. www.guysandstthomas.nhs.uk. Guy’s and St Thomas’ is part of King’s Health Partners Academic Health Sciences Centre (AHSC), a collaboration between King’s College London, and Guy’s and St Thomas’, King’s College Hospital and South London and Maudsley NHS Foundation Trusts. www.kingshealthpartners.org.
Tydeman® is a UK and European registered trademark of Guy’s and St Thomas’ NHS Foundation Trust. Patent protected (US 10219833 / AU 2016210033 / EP 3247292). Design rights (GB 4034383 / EU 002506881).
Media Contacts
Rocket Medical Plc.
Contact: Helen Grace and Jae Wyld
Email: heleng@rocketmedical.com, JaeWyld@rocketmedical.comPhone: +44 (0)191 419 4488
www.rocketmedical.com
Guy’s and St Thomas’ NHS Foundation Trust
Contact: Press Office
Email: press@gstt.nhs.uk
Phone: +44 (0)20 7188 5577
www.guysandstthomas.nhs.uk[1] Cornthwaite K, Bahl R, Lenguerrand E, Winter C, Kingdom J, Draycott T. Impacted foetal head at caesarean section: a national survey of practice and training. Journal of Obstetrics and Gynaecology. 2020;41(3):360–6.
[2]
NHS Resolution. The Early Notification scheme progress report: collaboration and improved experience for families. An overview of the scheme to date together with thematic analysis of a cohort of cases from year 1 of the scheme, 2017–2018. September 2019. https://resolution.nhs.uk/wp-content/uploads/2019/09/NHS-Resolution-Early-Notification-report.pdf.
[3] Vousden N, Tydeman G, Briley A, Seed PT, Shennan AH. Assessment of a vaginal device for delivery of the impacted fetal head at caesarean section. JOG 2017; 37(2): 157-61.
[4] Cornthwaite, K., Draycott, T., Bahl, R., Hotton, E., Winter, C., & Lenguerrand, E. (2021). Impacted fetal head: A retrospective cohort study of emergency caesarean section. European journal of obstetrics, gynecology, and reproductive biology, 261, 85–91.
Distributed by https://pressat.co.uk/